1 exhibit cubic symmetry which is characterized among other things by a three fold rotation axis along the space diagonal from the viewpoint of thermal conductivity and other properties based on second order tensors cubic materials behave as if they were isotropic according to neumann.
Cubic unit cells in ceramics.
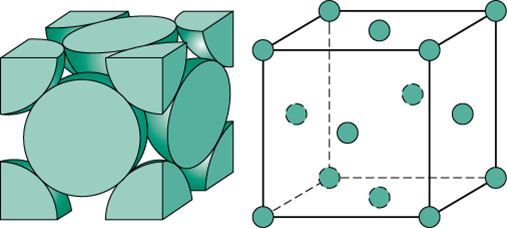
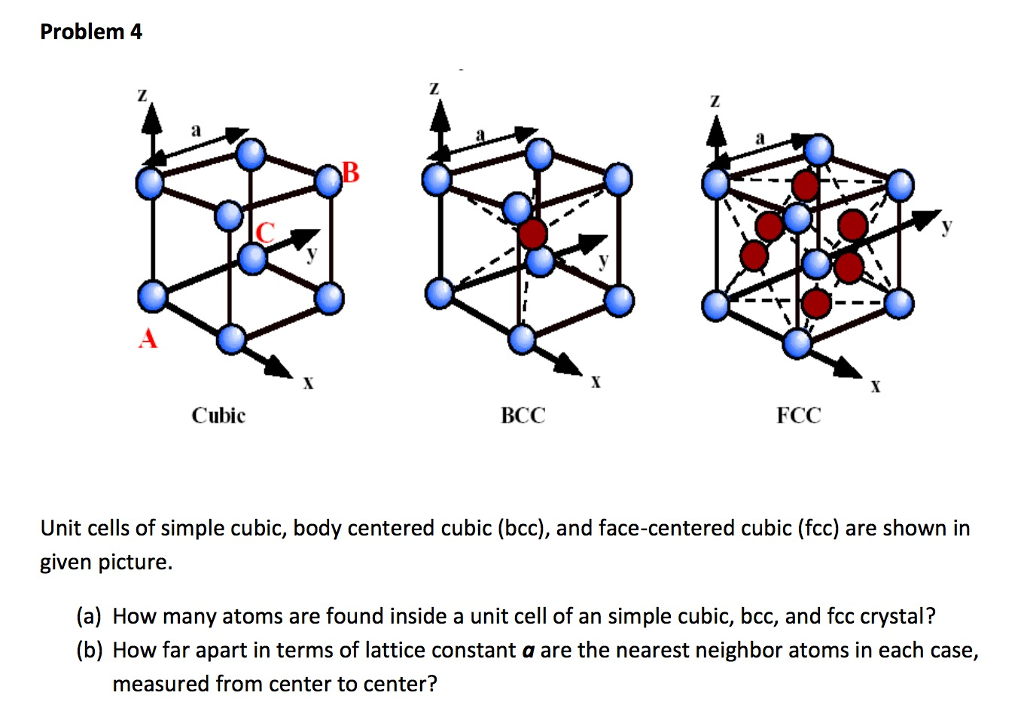
A body centered cubic unit cell has four atoms per unit cell.
A three dimensional graph.
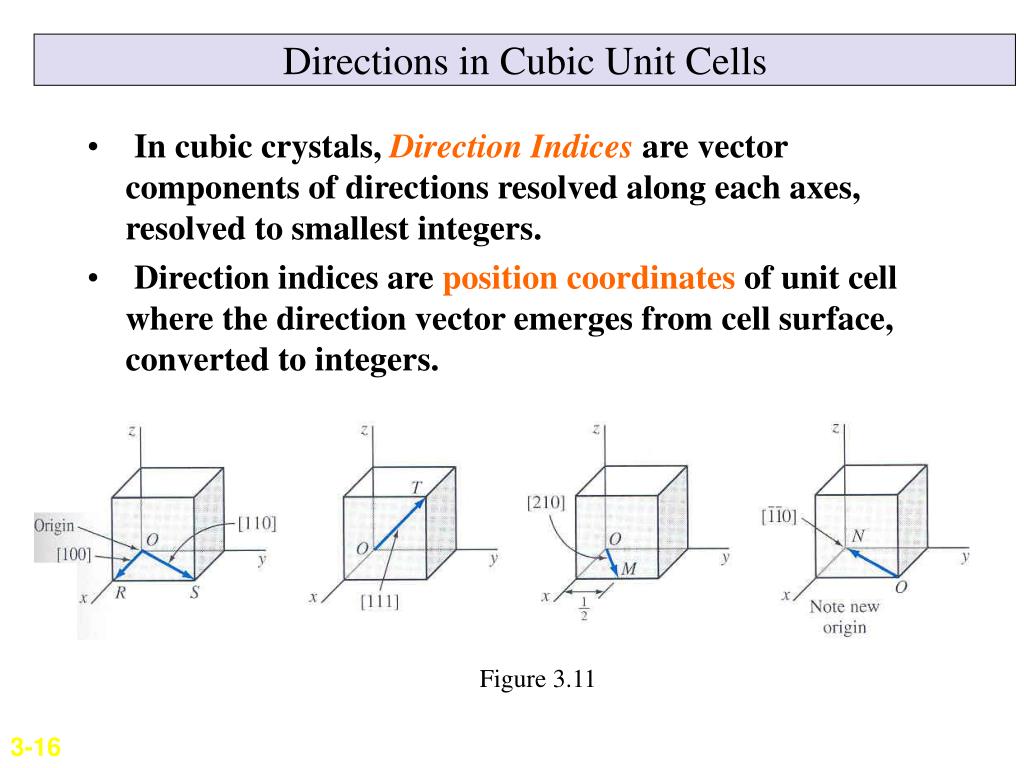
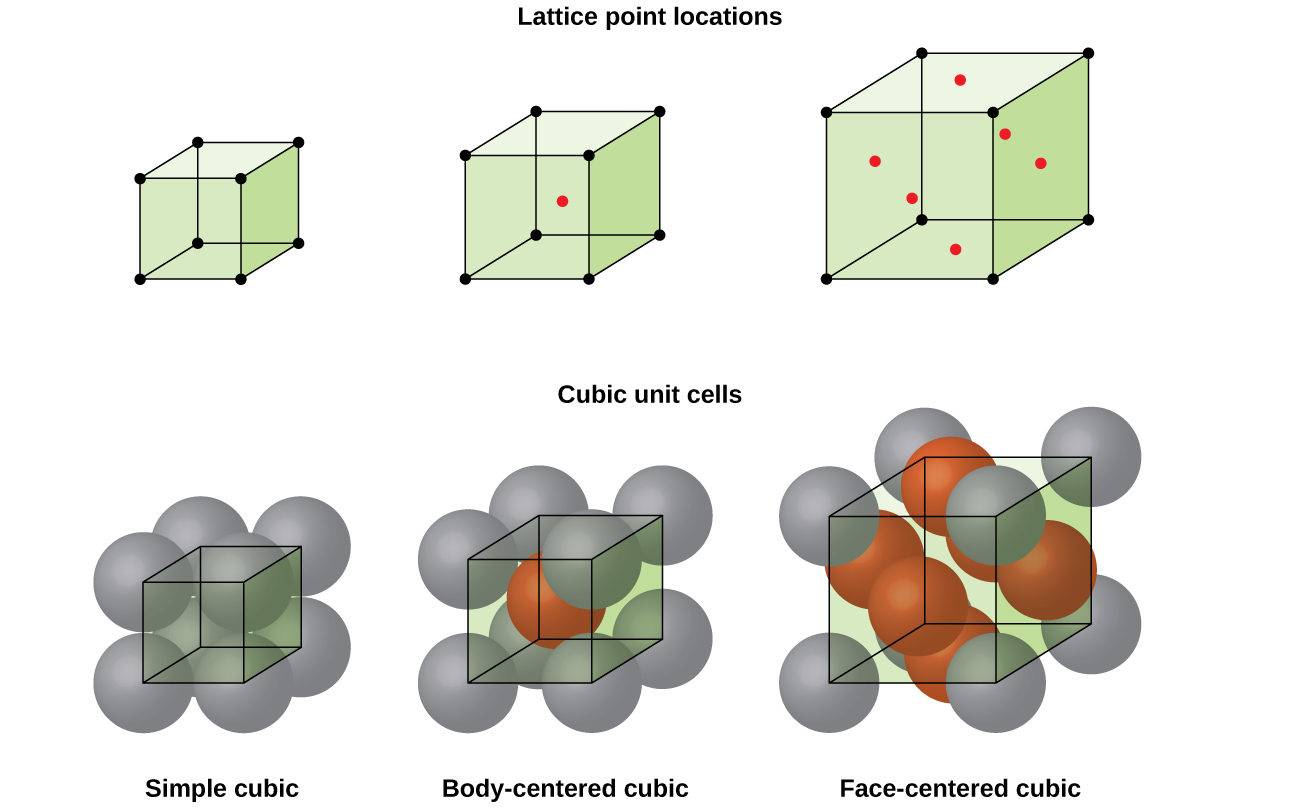
The lattice points in a cubic unit cell can be described in terms of a three dimensional graph.
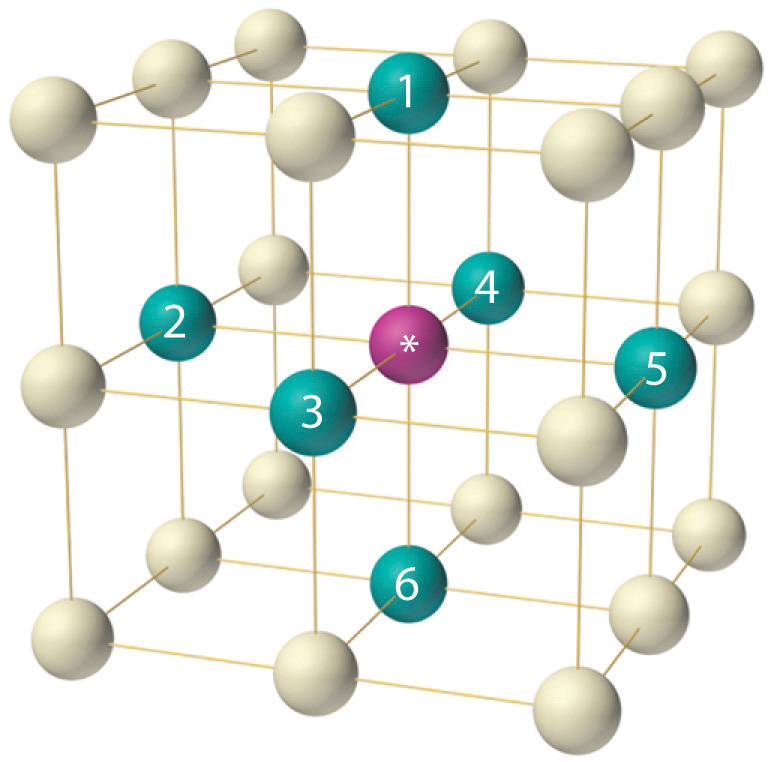
Body centered cubic bcc structure the body centered cubic unit cell has atoms at each of the eight corners of a cube like the cubic unit cell plus one atom in the center of the cube left image below.
The diagram to the right shows edges for an equivalent unit cell with a.
Each of the corner atoms is the corner of another cube so the corner atoms are shared among eight unit cells.
Pseudo cubic 0 64bf 0 36bt lead free ceramics were studied exhibiting high electric field induced strain of 0 38 60 kv cm with large signal piezoelectric coefficient d 33 of 720 pm v 40 kv cm being about 40 higher than those of other.
Cellular materials with a simple cubic spatial array of cubic unit cells either closed or open see fig.
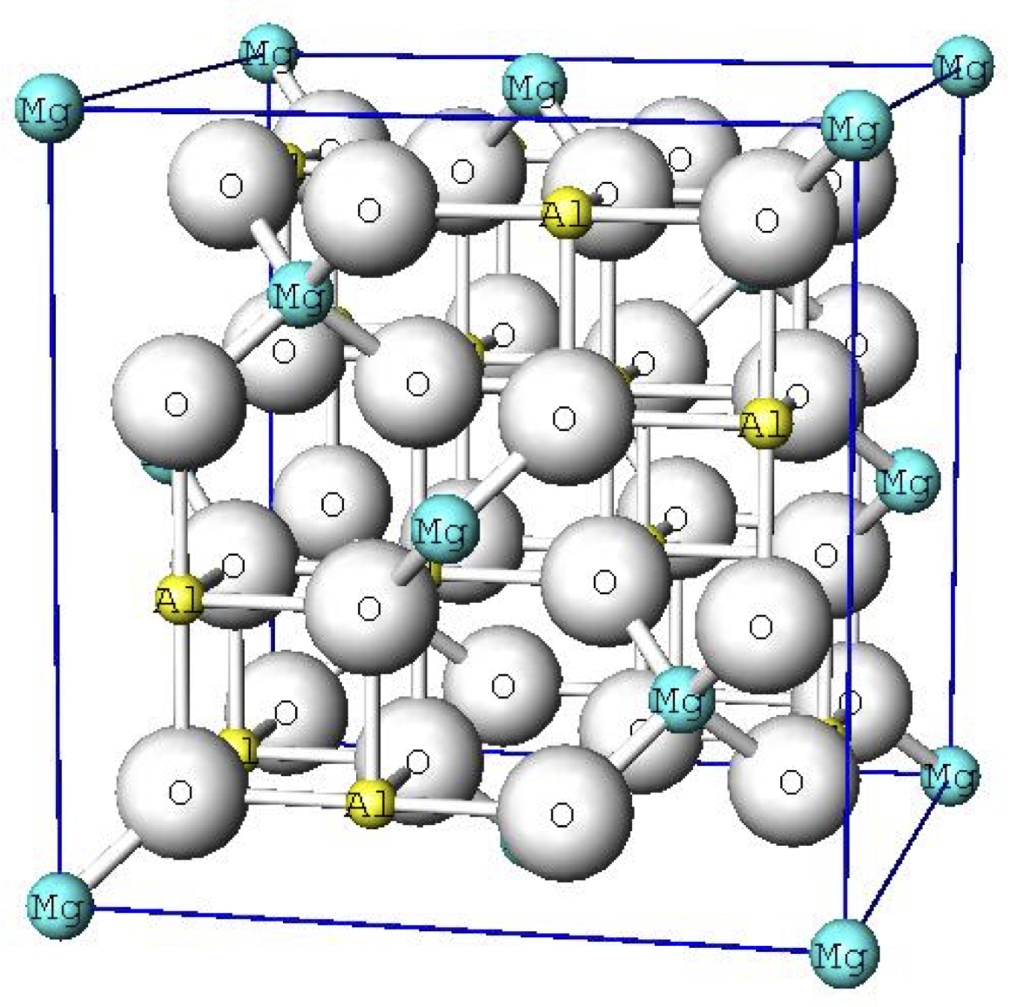
Unit cell consists of 8 cubes a unit cell of caf2 ax2 the ambnxp type crystal structures ba at cubic corner o at center of 6 faces ti at body center cn o 12 cn ba 6 and cn ti 6 large a cation and oxygen form an fcc lattice cubic tetragonal at 1300c curie points cubic orthrhombic and rhombohedral at low t a unit cell of perovskite.
Unit cell volume 16r3 2 avogadro s number 6 02 x 10 23 atoms mol r 8 89 g cm3 measured value 8 94 g cm 3 density mass volume ρ na v cn a n number of atoms unit cell a atomic weight v c volume per unit cell n a avogadro s number atomic packing factor fraction of solid sphere volume in a unit cell e g.
Cations in cubic sites uo 2 tho 2 zro 2 ceo 2.
In the idealized cubic unit cell of such a compound the type a atom sits at cube corner position 0 0 0 the type b atom sits at the body center position 1 2 1 2 1 2 and oxygen atoms sit at face centered positions 1 2 1 2 0 1 2 0 1 2 and 0 1 2 1 2.
If for a specific ceramic each unit cell has 6 cations and the cations prefer oh sites 4 in oh 2 in td.
For unit cells having a simple cubic primitive structure there would be one atom net for each unit cell.
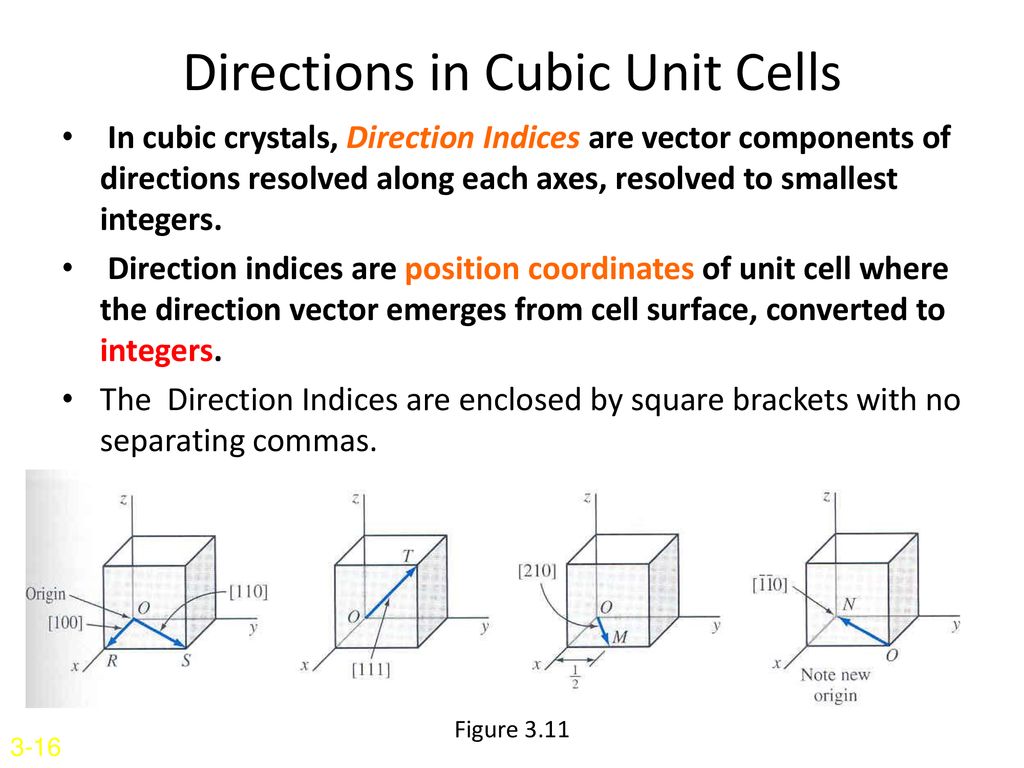
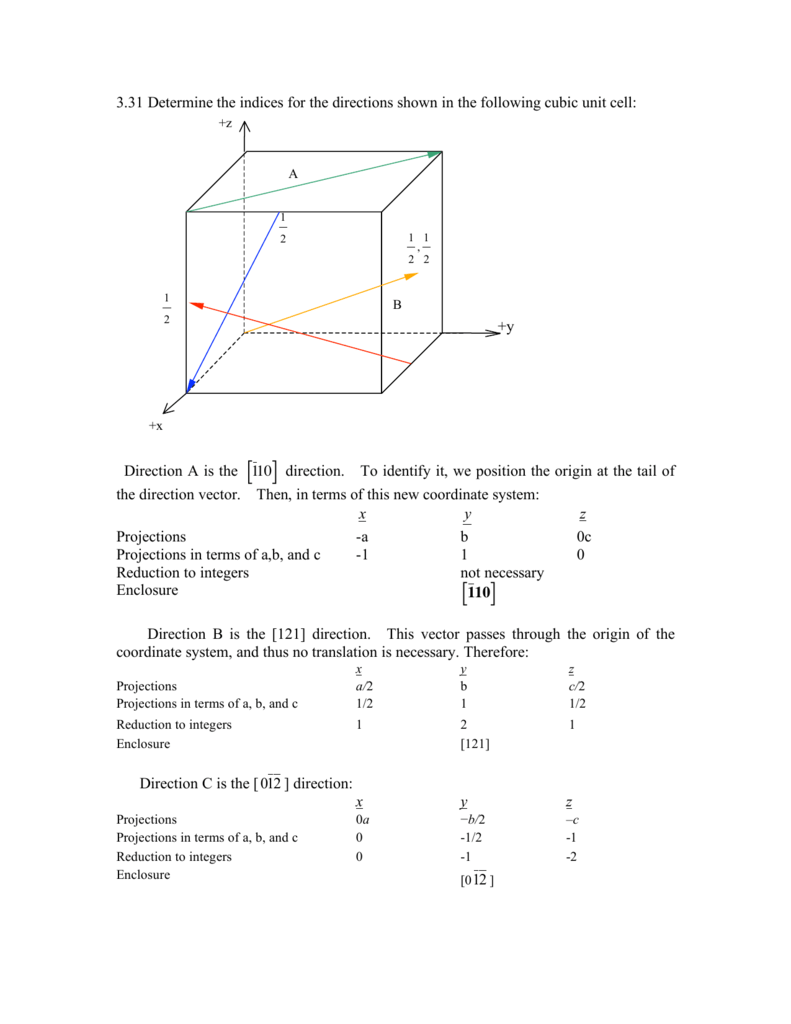
For the sake of argument we ll define the a axis as the vertical axis of our coordinate system as shown in the figure.
A unipolar s e curves of 0 64bf 0 36bt ceramics under different electric fields b 222 pc reflections with the increase of electric field.
For some repeating volume calculate the volume of the atoms inside and divide by the total volume.
Atoms in a solid consisting of only one element would have six nearest neighbors if the crystal structure were a simple cubic array.
11 interstitial sites in fcc 1 at the center 12 middle of the edge sites each shared by 4 unit net 4 o h sites unit cell cells.
Because all three cell edge lengths are the same in a cubic unit cell it doesn t matter what orientation is used for the a b and c axes.












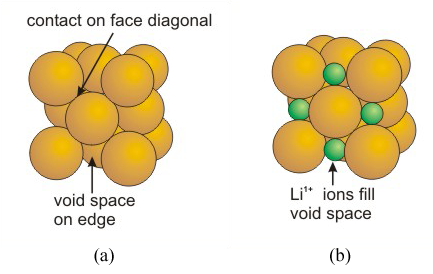



_1444200000_5614be4081c97_141196-17.jpg)